Useful Informations
Possible to prevent mycoplasma contamination

Biosafety Control Test
Biopharmaceuticals are mainly produced by using animal cell culture technology. Because Biopharmaceuticals are administered directly to patients, it is highly important to eliminate biological impurities derived from animal cells.
To detect mycoplasma, adventitious viruses, and host cell residual DNA, which are existent in biomedicine in small quantities, the highest PCR technology is required.
Why is Mycoplasma Contamination Important?
Contamination of cell cultures disrupts the physiology and metabolism of cells. Due to contaminated cell cultures, the efficiency of research and industrial studies is also adversely affected. In addition to preventing the accurate diagnosis of diseases, contamination in the cell cultures where virus vaccines are prepared poses also a serious danger to human health.
Mycoplasmas are the smallest organisms that can divide and multiply spontaneously. In general, while mycoplasmas prevent cells from growing in a healthy way, they can cause many different problems in cultured cells, including chromosomal abnormalities. Since they are generally not detectable with the naked eye and are quite common, Mycoplasma contamination is a significant threat in cell culture studies.
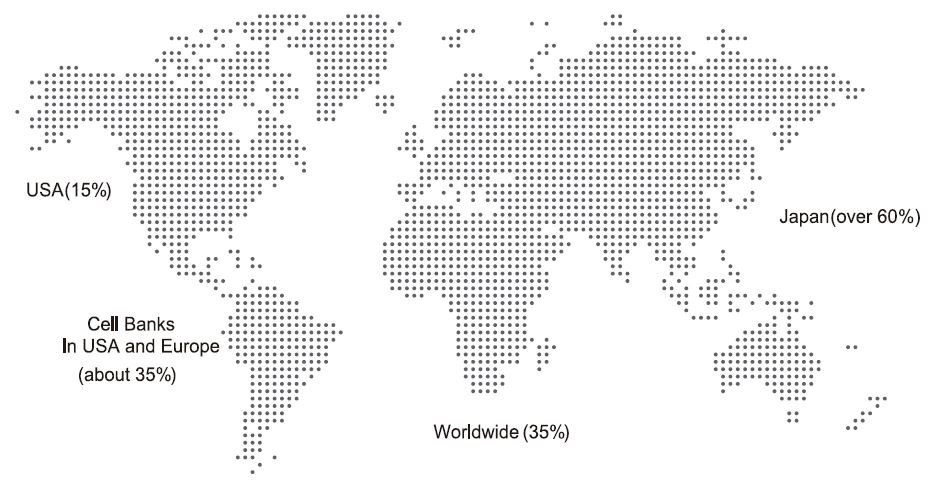
Distribution of Mycoplasma Contamination In Cell Culture
Cell Culture and Mycoplasma
Mycoplasma is the smallest, a mollicute genus bacteria that lack of a cell wall around their cell membranes. There are about 209 known species. They are the major contaminant in research and production of biopharmaceuticals using animal cell culture. Mycoplasma contamination in cell culture causes severe problems;
-Inaccurate or erroneous experimental results
-Loss of time, money and effort
-Reduction in productivity
-Lower the product quality
Therefore periodic management is essential because mycoplasma contamination can’t be observed.
Most common mycoplasma species in cell culture (origin) are;
-20-40% M.orale (human)
-10-40% M.hyorhinis (pig)
-20-30% M.arginini (cow)
-10-20% M.fermentans (human)
-10-20% M.hominis (human)
-5-20% A.laidlawii (cow)
CellSafe Mycoplasma detection Kit Line-Up
|
Product Type |
Conventional PCR | Real-Time PCR | ||
|
MycoQsearchTM
|
||||
| Application | For accurate Mycoplasma detection | For accurate Mycoplasma detection | For accurate Mycoplasma detection | For accurate Mycoplasma detection |
| Method | Conventional PCR | Conventional PCR | qPCR-Probe Type | qPCR-Probe Type |
| Detection Limit | 10fg ~ 100fg/reaction | 1fg ~ 10fg/reaction | 10fg ~ 100fg/reaction | 1fg ~ 10fg/reaction |
| PCR reaction time | 1 hr 10 min | 1 hr 30 min | 1 hr 10 min | 1 hr 30 min |
| Validation | Korea MFDS EP 2,6,7 | Korea MFDS EP 2,6,7 | ||
| Interest Group | R & D | ATMP* manufacturer, Pharmaceutical Industry | R & D | ATMP* manufacturer, Pharmaceutical Industry |
*Advanced Therapies Medicinal Products